European (Regulation EU 2017/745) and worldwide marketing of your medical devices (MD)
The Emitech Group is a major player in tests that apply to equipment. In the medical sector, our services are intended for manufacturers, importers and distributors of medical devices (MD), laboratory equipment and paramedical equipment.
MDs are governed by specific regulations throughout the world.
We can give you an overview of the classification of your MDs, and inform you of what this entails in terms of procedures, testing requirements, and the documentation to be compiled. We can then guide you through each step of the process through to the marketing of your products.
Compliance with the tests conditions the marketing process
To be approved in Europe (CE marking) and in the rest of the world, Medical Device must conform to tests covering multiple fields: electromagnetic compatibility (EMC), radio, EMF (electromagnetic waves & health), safety or mechanical.
As the leader in environmental testing, the Emitech Group can offer you its expertise in all of these fields.
From the start of our discussions, we can identify the tests to which you will be subjected and their severity, according to the countries targeted by your market launches.
Emission DC - 40 GHz
One measuring line per room and open area test site
- Radiated emission (Measurement at 3 m in Full Anechoic Room or at 3 or 10 m in large semi-anechoic chamber or in open area test site from 30 MHz to 26 GHz)
- Conducted emission (from power supply, single phase LISN 10 A, three phase LISN 100 A, three phase LISN 400 A, rail of 1 to 6 meters cable slide bar for disturbing power emission.
Immunity 10 kHz to 6 GHz
One immunity line per room
- Radiated immunity (semi anechoic chamber from 27 MHz to 6 GHz or Full Anechoic Room from 80 MHz to 6 GHz)
- Conducted immunity (Inducted field strength 10Veff from 150kHz to 230MHz)
- Magnetic immunity (Magnetic field immersion method 30A/m_50/60Hz)
Electrical testing
A complete range of facilities for testing according to EN 61000-4-(2, 4, 5, 8, 9, 10, 11, ...) and EN 61000-3-(2 et 3) - EN 61100-3-(11 et 12)
- Electrostatic discharge up to 30kV - Electrical Fast Transient / Burst Immunity test: CDN 3 phases 30A, amplitude burst 4.4kV, CDN 3 phases 100A, amplitude burst 4.4kV - Surge immunity test: CDN 3 phases 25A amplitude 6.6 KV, CDN 3 phases 150A amplitude 6.6 KV, Voltage dips, short interruptions, and voltage variations
- Flickers and harmonics, Monophase and 3 phases 16 A, Monophase and 3 phases 32A)
- Air-conditioned rooms
- Controlled sources of electrical power (stabilised 50/60 Hz power supplies, generators / converters)
- Acquisition units for temperature rise measurements (thermocouples)
- Appliances for electrical measurements (oscilloscope, probes, multimeters, leakage currents, etc.)
- Appliances for power measurements
- Appliances for insulation measurements (dielectric strength meter, insulation strength)
- Appliance for ground continuity measurements
- Residual lightning impulse generating bay
- Transformer splitting machine
- Equipment for incandescent wire tests and for flammability tests
- Environmental chambers
- IP testing equipment
- Standardised gauges and templates (test fingers, etc.)
- Mechanical testing of electrical enclosures (shock hammers, indentors, etc.)
- Calm air chamber (luminaires)
- Automated systems and specific test fixtures
- Protection against electric shock
- Dielectric strength
- Temperature rise
- Leakage currents
- Insulation resistance
- Leakage paths and creepage distance
- CTI / Tracking
- Moisture resistance
- Danger from electromagnetic waves
- Incandescent wire
- Fire resistance
- Impact resistance
- Drop tests
- Fault condition analysis
- Liquid ingress protection (IPX1 to X8)
- Solid ingress protection (IP1X to 6X)
- Protection against external mechanical impacts (IK01 to IK10)
- 10)
- Open area test sites for mesures at 10m
- Full Anechoic Rooms for measurement at 3m test distance
A Faraday room with ferrite tiles and pyramidal absorbers builded to be a full anechoic room can comply the site attenuation of an open area test site.
The emission measurements are equivalent and have the advantage to be also insulated from exterior sources of electromagnetic noise.
We obtain faster measurments in FAR
- Large Semi-Anechoic Chambre for measurements at 3 or 10m test distance
- Emission measurement equipment up to 40GHz:
- analysers,
- antennae,
- pramplifiers,
- cables,
- etc.
- Radiocommunication bench
- Analog and digital modulation analysers (GSM)
- Protocole analysers
- Climatic chambers
Open area test site

Full Anechoic Room

Semi-Anechoic Chamber

- IEC 60601-1 / EN 60601-1 # Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
- IEC 60601-2-XX / EN 60601-2-XX / IEC 80601-2-XX / EN 60801-2-XX # Medical electrical equipment - Part 2-xx: Particular requirements for safety
- IEC 60601-1-XX / EN 60601-1-XX # Medical electrical equipment - Part 1: Particular requirements for collateral standards
- Deviation analysis by country (example: UL 60601-1 / AAMI ES60601-1 / CSA C22.2 No. 601.1, deviation for North American countries)
The 60601 series of standards includes 60601-1 and 60601-2-XX / 80601-2XX. Part 1 applies to all medical products, while Part 2 deals with the specific requirements for a particular type of medical device. There are more than 40 parts 2 (60601-2-XX, 80601-2-XX).
Standards 60601-1-X cover special tests such as those related to requirements for electromagnetic compatibility, radiation...
When products are too large or heavy to be evaluated in our laboratories, we can perform in-situ tests anywhere in the world and in the most specific time slots.
- IEC 60601-1-2 / EN 60601-1-2 # Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic disturbances
- IEC 60601-2-XX / EN 60601-2-XX # Medical electrical equipment - Part 1-2: Particular requirements
Our global approach to your medical devices
Expertise
- Characterisation of medical devices (MDs)
- Validation of the applicable directives (medical directives + any additional directives)
- Classification of the MD according to the 18 rules defining the risk levels (I, IIa, IIb and III)
- Implementation of the regulatory strategy
- Consistency of the classification in relation to the study stemming from the risk analysis / improvement of software reliability and applicable standards
- List of the conformity items to be obtained
Testing
- Performance of the testing programme arising from the applicable standards for medical directives
- Basic standard IEC 60 601-1
Collateral standards 60 601-1-X
2 – electromagnetic compatibility,
4 - software life cycle (IEC 62304),
6 - usability for use (IEC 62366-1),
9 – environmentally conscious design,
10 – development of physiologic closed-loop controllers
11 – home healthcare environment
12 – emergency medical services environment
...
- Specific standards 60601-2-X / 80601-2-X
10 – nerve and muscle stimulators,
18 – endoscopic equipment,
49 – multifonction patient monitoring
52 – medical beds
57 – non-laser light source equipment
77 – robotically assisted surgical equipment
...
- For complementary directives
- Radio standards, e.g. for the RED Directive
Assistance
- CE marking
- Direct use of test report for Class I MDs
Self-certification process
- Submission of test reports to the Notified Body from Class II
Certifcation process
- Assistance in drafting the EC technical file, regulatory support, demonstration of conformity with essential performance requirements.
- CB scheme
Approved by the Emitech Certification Body, the reports are your gateway to more than 50 countries
- Approval for a series of destinations
Bespoke support for selling throughout the world
Access to the main marks of conformity (NRTL, ...)
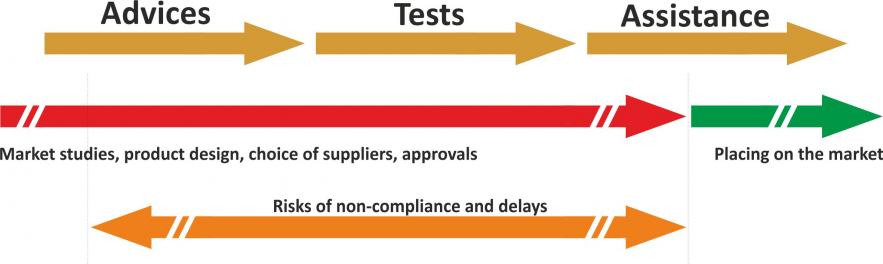
Protect your brand image
Differentiating services are also offered in mechanical and climatic environments and in reliability, with the objective of bringing to market more robust and tested products to operate longer or simply to be transported to the other end of the world without deterioration (packaging).